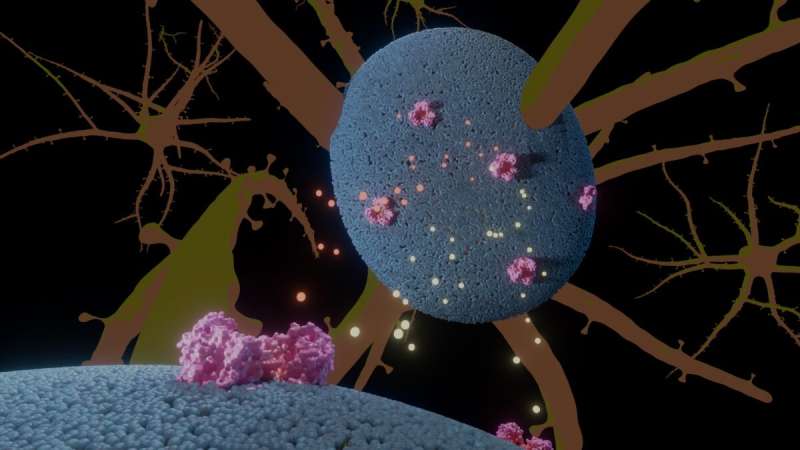
Illustration of the glutamate transporter (pink) in neural cells (blue), with glutamate and anions in yellow and orange. Credit: A. Guskov, University of Groningen
Worldwide, just a handful of clients are understood to struggle with episodic ataxia type 6, a neurological illness that triggers short-term loss of muscle control. The cause depends on an anomaly that alters a single amino acid in a protein that carries the neurotransmitter glutamate throughout the membrane of neural cells. Scientists from the University of Groningen (the Netherlands) have actually clarified how the anomaly triggers these cells to breakdown. Their outcomes appear in Nature Communications
Clients with ataxia lose control of their muscles, which can for instance impact how they move or talk. A very unusual kind of this illness is episodic ataxia type 6 (EA6), in which clients suffer episodes of ataxia. Worldwide, there are simply over a lots recognized clients, consisting of one household in the Netherlands. It is understood that EA6 is triggered by a single anomaly, however how this anomaly can have such a remarkable impact was so far a secret.
“This protein carries glutamate throughout the membrane of neural cells,” describes structural biologist Albert Guskov. The protein is placed in the cell membrane, and the anomaly alters a proline amino acid in among the helical transmembrane domains into an arginine.
“A proline in a helix normally triggers a kink,” describes Guskov. “If a proline is become an arginine, we would anticipate this kink to vanish. To check this, we studied the structure of the altered protein.”
Given that the human transportation protein is hard to study in the laboratory, Guskov and his associates utilized a comparable protein from archaea, an ancient type of unicellular organism. “This archaeal protein has actually been well saved throughout advancement, and we understand from previous work that it is an excellent design for the human transportation protein, although it carries aspartate and not glutamate,” discusses Guskov.
Utilizing cryo-electron microscopy on regular and altered proteins put in lipid nanodiscs, the group had the ability to compare the shape of the altered protein to the typical variation. In previous research studies, the group had actually revealed that part of the protein goes up and down through the membrane, similar to an elevator. The hypothesis was that the anomaly would trigger the transmembrane kink in the protein to vanish, which this would alter the protein’s shape and obstruct the elevator motion.
That was not the case. Gustov states, “To our surprise, the kink was still there.” The anomaly did impact the performance of the protein. “The transportation rate was minimized by an element of 2, compared to the regular protein.” Throughout transportation of the aspartate, the protein transiently formed an anion channel. “And in the altered protein, ion transportation was 3 times greater.”
In some way, the arginine that changed the proline did not change the shape of the transportation protein, however it did impact its function. The scientists carried out molecular characteristics simulations, which reveal all the interactions of the amino acids of the protein with their environments. “What we discovered is that a salt bridge is formed in between the arginine amino acid and the lipids of the membrane.” This salt bridge, a kind of tourist attraction in between particles, appears to decrease the motion of the elevator part of the protein.
Gustov states, “If this elevator moves more gradually, it discusses the reduction in aspartate transportation, however it likewise implies the short-term ion channel stays open longer, hence allowing more anions to travel through.” In human neural cells, this would result in a lowered transportation of the neurotransmitter glutamate, and increased anion imbalance. These findings discuss how this anomaly triggers ataxia. “Both have really nasty effects for the performance of neural cells.”
There is no easy method to treat the result of the anomaly. Gustov states, “Furthermore, this transporter exists throughout the body, so any drug impacting it will most likely have major negative effects.” Considering that there are just a handful of clients, no drug business would invest in a remedy. “Although there may be a lot more clients. Given that it is an episodic disease and the signs can be moderate, lots of people may not know it. They are merely utilized to feeling weak for a couple of days at a time, much like somebody who experiences migraine.”
For the clinical neighborhood, these findings raise a variety of interesting concerns. Gustov states, “The protein has actually been effectively saved throughout evolutionary history. Why did this short-term anion channel appear, and has it turned out to be so helpful for archaea that it was brought over time best to our own nerve cells? That is what we want to comprehend.”
More details:
Emanuela Colucci et al, Mutation in glutamate transporter homologue GltTk offers insights into pathologic system of episodic ataxia 6, Nature Communications (2023 ). DOI: 10.1038/ s41467-023-37503-y
Citation: Basic science demonstrates how a single anomaly triggers ataxia (2023, April 3) obtained 3 April 2023 from https://phys.org/news/2023-04-basic-science-mutation-ataxia.html
This file undergoes copyright. Apart from any reasonable dealing for the function of personal research study or research study, no part might be recreated without the composed consent. The material is attended to details functions just.